Detailed Steps For Government Oral Care Product Procurement
Introduction
The procurement of oral care products by government agencies requires careful consideration of industry regulations, supplier compliance, and effective decision-making. Government procurement processes are often governed by strict guidelines and timelines to ensure that public funds are spent efficiently and effectively. As a key player in the oral care manufacturing industry, understanding these processes is essential for manufacturers and suppliers who aim to work with governmental bodies.
This article provides an in-depth overview of the government procurement process for oral care products, offering key insights and actionable strategies for procurement personnel. By understanding the procurement steps, timeframes, and compliance requirements, government officials can make informed decisions that benefit both the public sector and the people it serves.
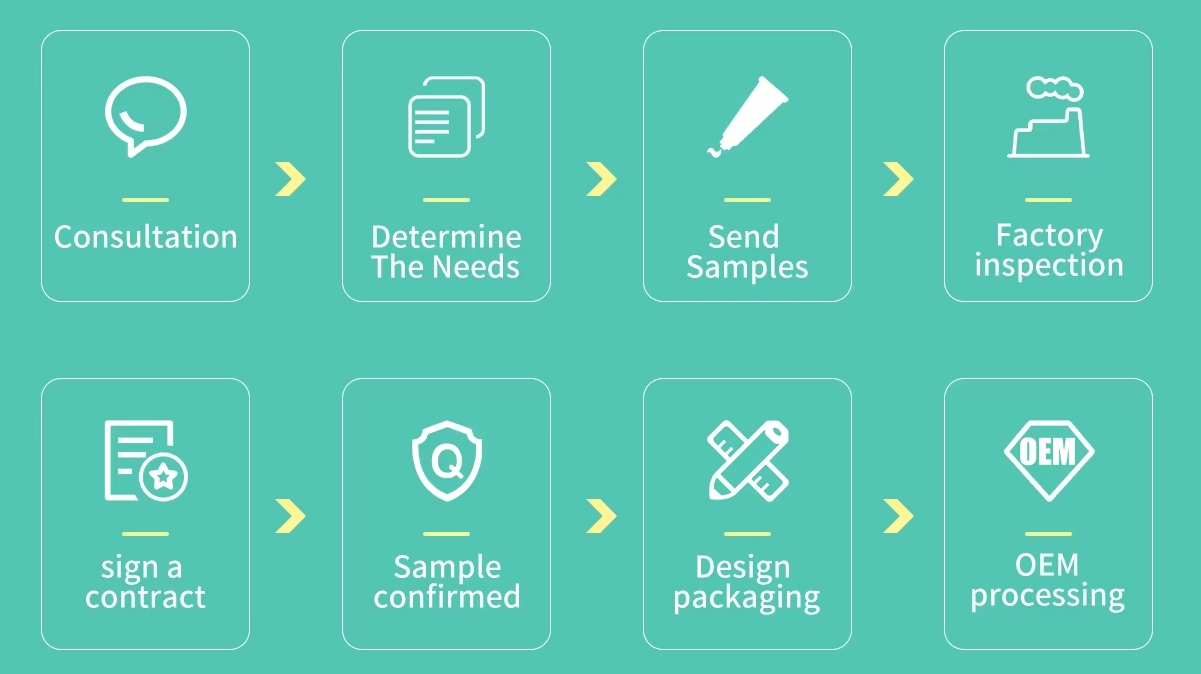
1. Initiating the Procurement Process: Needs Assessment
The first step in any government procurement process is identifying the need for specific products. For oral care products, this could range from basic toothbrushes and toothpaste to more specialized items such as fluoride treatments, mouthwash, and dental hygiene kits for schools, hospitals, or prisons.
Key Considerations:
- Product specifications: Government agencies must define the precise specifications of the oral care products they need. This includes understanding factors such as product quality, sustainability, and any special certifications required.
- Stakeholder input: The procurement team should consult with relevant departments or experts to ensure that the products meet health and safety standards, as well as the end users’ needs.
2. Developing a Request for Proposal (RFP)
Once the need for oral care products has been established, the next step is to develop a Request for Proposal (RFP). An RFP is a formal document that outlines the requirements, timeline, and expectations for potential suppliers.
Key Elements of an RFP:
- Detailed product specifications: Exact requirements, including quality standards, certifications (e.g., FDA, GMP), and labeling.
- Supplier qualifications: Information on the supplier’s ability to meet the requirements, such as factory certifications, past government contracts, and compliance with local regulations.
- Delivery timelines: Government procurement processes often have strict delivery deadlines, which need to be clearly outlined.
- Cost and payment terms: A transparent cost structure, including any applicable taxes or shipping fees.
The RFP process helps ensure that only qualified suppliers are considered for the procurement opportunity.
3. Supplier Evaluation and Selection
After the RFP has been sent out to potential suppliers, the next step is the evaluation phase. Government procurement personnel must assess each supplier’s proposal based on several criteria, including cost, product quality, delivery timelines, and compliance with regulations.
Key Evaluation Criteria:
- Regulatory compliance: The supplier’s ability to meet all industry regulations, such as GMP (Good Manufacturing Practices) and ISO certifications.
- Product quality assurance: Manufacturers must provide proof of product testing and safety standards. Any oral care products sold to government agencies must be of the highest quality.
- Sustainability: With growing attention on eco-friendly products, sustainability in manufacturing and packaging can be an important evaluation factor.
- Cost-effectiveness: Although cost is an essential factor, the lowest bidder is not always the best choice. Long-term value and quality should be prioritized.
4. Negotiation and Contract Awarding
Once the evaluation is complete, the next step involves negotiating the terms of the contract with the selected supplier. This stage involves finalizing the prices, delivery timelines, payment terms, and any other contractual obligations.
Key Considerations in Negotiation:
- Compliance with laws and regulations: Ensure that the contract aligns with local, regional, and international regulations, including safety and ethical standards.
- Price negotiations: While price is crucial, it should never be at the cost of quality. Procurement personnel should work to balance affordability and quality.
- Delivery schedules: Establish clear delivery timelines, especially if the oral care products are required for specific events, like school programs or health initiatives.
- Penalties and incentives: The contract should include provisions for penalties in case of delays or non-compliance, as well as incentives for early or on-time delivery.
5. Product Inspection and Quality Control
Before the final acceptance of the oral care products, governments often require a comprehensive product inspection and quality control process. This step ensures that the products meet the agreed-upon specifications and comply with safety standards.
Key Actions:
- Third-party testing: Government procurement teams may require third-party verification of product safety, especially for health-related items like oral care products.
- Sampling and inspection: Regular product sampling and inspections may be conducted to ensure the products’ consistency and quality throughout the production process.
- Documentation and reporting: Manufacturers are required to provide certificates of compliance, test reports, and other documentation to verify the products’ safety and quality.
6. Delivery and Post-Delivery Services
Once the contract has been awarded and the products pass the quality inspection, the delivery phase begins. This is when the supplier delivers the products to the designated government location, which could be a warehouse, hospital, or public health office.
Key Steps in Delivery:
- Timely delivery: Government procurement contracts typically specify strict delivery deadlines. Ensuring on-time delivery is crucial to maintaining compliance.
- Post-delivery support: Suppliers should be prepared to provide post-delivery services, such as product installation (if applicable), maintenance, and handling returns or complaints.
7. Ensuring Ongoing Compliance
After the procurement process is completed, ongoing monitoring is essential to ensure the supplier continues to meet regulatory standards and product quality expectations. This involves conducting routine inspections, performance reviews, and audits.
Ongoing Compliance Checks:
- Regulatory updates: Keep track of any changes in industry regulations, such as new health guidelines or manufacturing standards.
- Supplier audits: Regular audits may be conducted to ensure suppliers are adhering to contractual terms and meeting quality standards.
- Feedback loops: Government agencies should have mechanisms in place to collect feedback from the end-users of the products (e.g., hospitals, schools) to ensure the products meet their needs.
Try Lidercare Now!
We Help You Launch New Products, And Continue To Grow. Try Us With 20% Off Your First Order!
Conclusion: Making Informed Procurement Decisions
Navigating the government oral care procurement process can be complex, but understanding the key steps, timeframes, and compliance requirements helps procurement teams make better decisions. By ensuring that all regulations are met, selecting the right suppliers, and following through with proper post-delivery support, government agencies can secure high-quality oral care products that benefit the public.
Why Partner with Lidercare?
As an industry leader in the oral care manufacturing space, Lidercare is committed to delivering high-quality, compliant products that meet government standards. Our FDA-certified facilities and strict adherence to Good Manufacturing Practices (GMP) make us a trusted partner for government procurement teams.
Key Advantages of Partnering with Lidercare:
- Regulatory Compliance: We ensure all products meet local and international standards.
- Customizable Solutions: Whether you need private-label options or tailored formulations, we offer flexible solutions.
- Timely Delivery: Our robust supply chain ensures on-time delivery, every time.
- Post-Delivery Support: We provide excellent after-sales service, including product guarantees and customer support.
Let Lidercare be your trusted partner for high-quality, reliable oral care products. Contact us today to learn more about how we can support your procurement needs.
FAQs:
1. What is the typical timeline for government oral care product procurement?
The procurement process typically spans several weeks to months, depending on the complexity of the tender. It includes steps like the needs assessment, RFP development, supplier selection, and contract negotiations.
2. What are the key factors to consider when selecting an oral care supplier for government contracts?
Key factors include regulatory compliance, product quality, supplier reliability, cost-effectiveness, and delivery timelines. Ensure the supplier is certified by relevant bodies such as the FDA and meets safety standards.
3. How can I ensure ongoing compliance with industry regulations post-procurement?
Regular audits, inspections, and monitoring of product performance are essential. Keeping up-to-date with changes in industry regulations and maintaining open communication with suppliers helps ensure continued compliance.
Table of Contents
Awesome! Share to:
Latest Blog Posts
Check out the latest industry trends and take inspiration from our updated blogs, giving you a fresh insight to help boost your business.